Participate - Portsmouth Hospitals NHS Trust
This site is recruiting participants from the Hampshire and Dorset area.
This site is not currently recruiting
Comparing COVID-19 Booster Vaccinations (COV-BOOST)
What is the purpose of this research trial?
There are now a number of vaccines that have been approved in the UK to prevent COVID-19 and other vaccines that are still in UK clinical trials but which may be approved later in the year. Millions of people have now received their first 2 vaccinations, which we call a “prime-boost” course. There were 2 vaccines used by the NHS to deliver an initial prime-boost during the first part of the NHS immunisation campaign: ChAdOx1-nCov19 (commonly known as the “Oxford vaccine”), and BNT1162b2 (commonly known as the “Pfizer vaccine”). Whilst these have been shown to be highly effective at preventing severe disease due to COVID-19, we don’t know how long the immune protection from vaccination will last. In addition, variants of the virus which causes COVID-19 (SARS-CoV-2) have emerged with mutations which might make the immune response from vaccination less effective. It is therefore likely that additional, “booster” vaccinations might be needed for high risk groups after a period of time to provide added protection. This study is trying to find out which vaccines against COVID-19 are most effective as a booster vaccination, depending on which vaccine was used to provide the initial prime-boost course. We will be enrolling men and women over the age of 30 who received their initial prime-boost course of vaccination against COVID-19 in December 2020, January or February 2021, and who have also received the second dose booster 70 days or more by the time people join this trial.
What are the vaccines against?
These vaccines are against the new coronavirus SARS-CoV2 that causes the disease COVID-19.
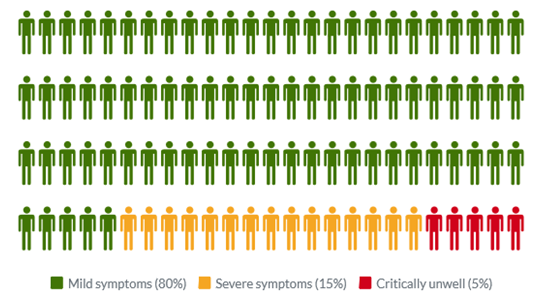
Common symptoms of COVID-19 include fever, tiredness, dry cough, and changes to taste and smell. Whilst about 80% of infected people have no or mild symptoms and will recover from the infection without needing special treatment, approximately 10-15% of cases (2-3 in 20) progress to develop severe symptoms, and about 5% (1 in 20) become critically ill.
There are some treatments that have been shown to be effective in reducing the severity of disease and the risk of death; but at present there is no cure. Older people and those with underlying medical conditions are more likely to develop serious illness. It has also been seen that people of some ethnic groups (Black and Asian) might be at a greater risk of severe illness. More than 2.5 million people globally have died from COVID-19 so far. Some people also have symptoms that last a long time after they have recovered (commonly referred to as “long-COVID”). This is why effective vaccines are so important.
Am I suitable to take part?
Adults that are aged 30 and over who received their first dose of COVID-19 vaccination in either December 2020, January or February 2021 and who are 84 days post second vaccination are able to take part (due to the NHS deployment timelines, some sites may need to invite people who have been prime-boosted with their second dose of AstraZeneca vaccine with a minimum of 70 days from their second dose). In order to be enrolled in the trial:
- You must be willing to tell the trial staff about your medical history, and you may be asked to allow the trial staff to check this with your General Practitioner (GP). Bear in mind that we would also notify your GP if you joined the trial (even if we did not need to check your medical history with them in advance).
- If you are able to become pregnant you must be willing to practice continuous effective contraception during the first 3 months of the trial and have negative pregnancy tests on the days of vaccination
- You must agree not to donate blood during the trial
For further details on why you could not take part in this study please see the Participant Information Booklet.
Summary of the study
We are studying combinations of seven different COVID-19 vaccines compared to a control group, who will be receive a vaccine against the meningococcal bacteria which causes meningitis and sepsis (Men ACWY). There will be 2886 participants.
As new SARS-CoV-2 vaccines become available, more vaccines may be included in the trial and so the total number of participants may increase (these will be in later stages of the trial).
Participants will be allocated, at random, (rather like a flip of a coin) to receive one dose of one of the seven study vaccines, or the control vaccine (MenACWY).
Four of the vaccines are currently approved by the MHRA for NHS use to prevent COVID-19, 1 is under review for approval by the MHRA and 2 are still in clinical trials but may be available in the UK later in 2021 if approved by the MHRA.
Between 4 and 6 routine blood tests will be taken over the course of a year to look at the immune responses to the vaccine depending on the group you are in. You may also be asked for a nasal fluid sample and a saliva sample at each visit. You might also be asked to attend for a repeat blood test if there were any safety concerns. If you were to test positive for the virus causing COVID-19 we may ask you to attend for an extra visit.
Participants will need to complete an online diary for up to 28 days following vaccination.
The trial will take one year to complete per participant (from the time the first dose of vaccine is given).
We would not be offering diagnostic COVID-19 testing as part of this trial, but it is important that participants in this trial access COVID-19 testing outside of the trial following normal government guidance.
You would not know which vaccine you had received until the end of the trial. Unless specifically advised by us, you would not be eligible to receive any further vaccine doses via the government vaccination scheme.
If you become eligible for a booster vaccination via the NHS during the course of the trial, we can find out whether you received a COVID-19 vaccine or the control vaccine (MenACWY). If you had not received a COVID-19 vaccine and you are eligible for one during any future NHS deployment, we will offer you one as part of the study.
If the vaccine you have been given in the trial is not found to be effective enough at providing a boost overall, and if your age group become eligible for NHS boost immunisation during 2021/2, then you will receive the approved NHS booster vaccine as part of the trial.
If you have received a vaccine that is considered effective you will remain in the trial for the remainder of the year with no further booster.
What vaccines are being used in this study?
The eight vaccines in this trial are ChadOx1 nCoV-19 (also known as AZD1222, developed in Oxford and manufactured by AstraZeneca), BNT162b2 (manufactured by Pfizer BioNTech), mRNA-1273 (manufactured by Moderna), NVX-CoV2373 (manufactured by Novavax), VLA2001 (manufactured by Valneva), CVnCoV (manufactured by Curevac), Ad26.COV2.S (Janssen) and the Meningococcal ACWY vaccine. We will also be trialling the Novavax, Valneva and Pfizer/BioNTech COVID-19 vaccines as a half dose. This is important as if this was effective it could allow for double the number of vaccinations to be given using the same vaccine supply. We are also studying whether a half dose of these vaccines causes less of the common vaccine side effects such as fever or sore arm while still providing adequate boost to the immune response.
By giving a booster dose with these vaccines, we are hoping to ensure that the body produces an immune response that will help provide extra protection against COVID-19 virus infections and disease. Because using different COVID-19 vaccines as a booster has never been tested before, we do not know if different vaccine combinations will provide an adequate level of increased protection.
None of these vaccines contain the live SARS-CoV2 virus and therefore cannot cause the infection.
What are the side effects of these vaccines?
Common side effects
People very often have tenderness, pain, warmth, redness, itching, swelling or bruising or less commonly have a small lump in their arm where they have been vaccinated.
Other common systemic side effects
Some people can develop these symptoms after vaccination. They usually last for less than a week after you are vaccinated (more commonly 24-48hours afterwards).
- Fatigue
- Headaches
- Flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills
- Muscle aches
- Joint aches
- Feeling unwell (malaise)
- Feeling sick or nauseated or vomiting
Other less common side effects:
- Abdominal pain
- Decreased appetite
- Feeling dizzy
- Swollen lymph nodes (glands)
- Excessive sweating, itching skin or rash
These symptoms can be reduced by use of paracetamol around the time of immunisation and over the next 24 hours.
After immunisation with the BNT162b2 (Pfizer/BioNTech) vaccine, difficulty sleeping has been observed in fewer than 1 in 100 people, and weakness of the muscles on one side of the face has been observed in fewer than 1 in 1000 people.
What are the advantages of taking part?
We anticipate that participating in the trial will mean that you gain some additional protection against the coronavirus if you were to be randomised to receive one of the COVID-19 vaccines (but cannot guarantee this). It is possible that you could gain this protection sooner than you otherwise would have by waiting for booster vaccinations to be recommended by the NHS. This would not be the case for the control group who receive the MenACWY vaccine. At this point it is not certain which age groups or risk groups will receive an NHS booster vaccine or when this will occur. Most importantly, the information gained from the trial will make a valuable contribution to the pandemic response.
Are there any risks from taking part in the study?
In addition to the potential side effects of the vaccines outlined above, the blood samples taken in the study may cause slight pain and occasionally bruising. Please refer to the participant information sheet for full details of procedures and potential risks.
What will happen if I don’t want to carry on with the trial?
If, at any time, after enrolment, you change your mind about being involved with this trial you are free to withdraw without giving a reason. If you withdraw we would not usually perform any more research procedures; although occasionally we might need to offer you a follow up visit for safety purposes, for example for blood tests. You would not have to agree to this. Your decision will not result in any penalty. Unless you state otherwise, any samples taken whilst you have been in the trial will continue to be stored and used for research as detailed above. You are free to request that your samples are destroyed at any time during or after the trial. Your data would be managed as laid out in the section ‘What will happen to my data’. If you choose to withdraw from the trial, your standard medical care will not be affected.
What’s next?
If you would like to find out more, please read the Participant Information Sheet and if you are interested in taking part, please complete our pre-screening questionnaire.
Download the Participant Information Sheet (PDF)
This site is not currently recruiting
Complete the Pre-screening Questionnaire
For further details contact us on :
or call 02392 892053




